Editor's note: Yellowstone Caldera Chronicles is a weekly column written by scientists and collaborators of the Yellowstone Volcano Observatory. This week's contribution is from Behnaz Hosseini, National Science Foundation Postdoctoral Research Fellow, and Madison Myers, Associate Professor of Volcanology and Geochemistry, both in the Department of Earth Sciences at Montana State University.
To time how fast a sprinter runs, you just need a stopwatch. To time how fast magma moves, you need to know a little bit about chemistry.
Picture a bizarre race: a sloth inching forward, a jogger picking up pace, an Olympic sprinter at full tilt…and deep underground, magma surging upwards. Who wins? Well, it depends. In some volcanic eruptions, magma creeps toward the surface so slowly that it would lose to the sloth, while in others, it would leave even Usain Bolt in the dust. But how do scientists measure a process that happens miles underground, hidden from view? The answer lies in tiny chemical changes preserved within erupted materials—microscopic clues that reveal the speeds at which magma rises toward the surface during explosive eruptions.
As magma cools, crystals begin to form—crystals that record changes in temperature, pressure, and chemistry within magma chambers. But crystals aren’t the only magma residents logging physical and chemical changes in their surrounding environment. Melt, the liquid portion of magma, also captures these changes! Enter embayments (also called reentrants), which are tubular melt-filled pockets burrowed into the side of volcanic crystals. Unlike melt inclusions, which are fully trapped inside crystals, embayments remain connected to the surrounding magma as the crystal forms, and so the embayments can record chemical shifts in the magmatic system up until the point of eruption. With the right tools, scientists can turn these tiny melt pockets into speedometers, clocking magma’s crawl (or sprint) to the surface.
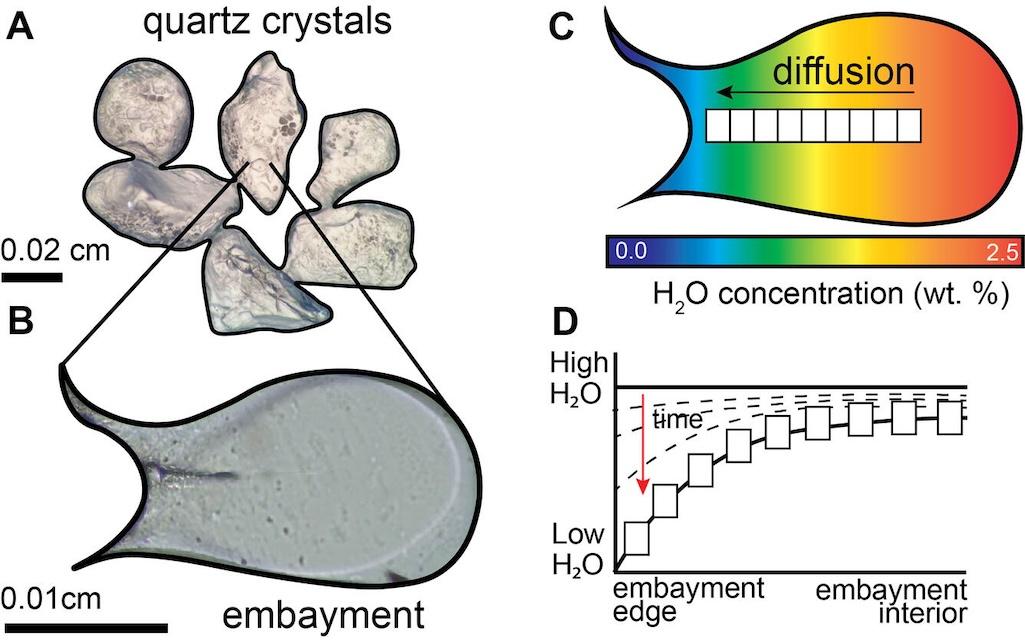
Quartz crystals (A) often contain melt embayments (tubular melt-filled pockets burrowed into the side of volcanic crystals) (B), which preserve volatiles (water, carbon dioxide, and sulfur) that have different concentrations in different parts of the embayment (C). The changes in concentrations within the embayments can be directly measured and modeled to infer the speed of magma as it ascended in the lead up to an eruption (D).
So, how does it work? It all comes down to the physical process of diffusion, which causes particles to spread from areas of high concentration to areas of low concentration—like a drop of food coloring dispersing through a glass of water over time. The same principle applies to chemical elements in melt. Volatile elements like water (H2O), carbon dioxide (CO2), and sulfur (S) are dissolved in melt under the high pressures within magma chambers. But as magma rises and pressure decreases, the concentration of these volatile elements also decreases as bubbles form. At high temperatures, this process drives diffusion of volatiles from the inner part of the embayment (where volatile concentrations are high) to the outer edge (where they are actively decreasing). The moment the magma erupts and completely solidifies, diffusion stops, locking a chemical “speed record” within the embayment. By measuring and modeling concentrations of volatile elements within embayments, scientists can determine how fast the magma was moving prior to eruption.
But how do scientists actually read these chemical “speed records”? That’s where specialized instruments and computer modeling come in! Using analytical techniques like Fourier Transform Infrared (FTIR) spectroscopy, scientists can precisely measure the concentrations of water and carbon dioxide trapped inside an embayment, creating a map of the chemistry from the outer edge to the interior. These profiles reveal how volatile elements degassed as pressure decreased during magma ascent, allowing scientists to work backward to calculate how quickly the magma traveled before it erupted.
Applying this technique to embayments in quartz crystals from Yellowstone’s massive Huckleberry Ridge Tuff eruption, which occurred 2.08 million years ago, scientists estimate that, upon the onset of eruption, magma ascended at rates ranging from 0.3 to 4 meters per second. This means it took anywhere from 10 minutes to 2 hours to travel from depths less than 10 miles to the surface—times that reflect a fully developed pathway for magma to rise once the eruption was well underway (the initial phases of pre-eruption magma rise would have been much slower). That’s astonishingly fast—faster than the average human’s long-distance running speed!

Range of speeds for several animals, athletes, and magmas from various volcanic eruptions. Eruptions shown include the 25,400-year-old Oruanui eruption of Taupo (New Zealand), the 2.08-million-year-old Huckleberry Ridge Tuff of Yellowstone (USA), and the 767,000-year-old Bishop Tuff of Long Valley (USA). Magma ascent rates determined by Myers et al. (2018). Figure by Behnaz Hosseini, Montana State University.
Measuring the ascent speed of magma isn’t simply an academic exercise—it has major implications for understanding how volcanoes like Yellowstone have behaved in the past. Whether magma rises at a sloth’s pace or races to the surface like a sprinter, the speed influences everything from the signals that monitoring systems like seismometers detect before an eruption to the style of eruption (for instance, explosive versus gentle). By unlocking the chemical records preserved in embayments, scientists can piece together the timing of past eruptions and, ultimately, improve how we monitor active volcanoes like Yellowstone. While we can’t witness magma’s race to the surface in real time, its chemical fingerprint preserved in embayments allows us to reconstruct its journey, one diffusion profile at a time.




 Support Essential Coverage of Essential Places
Support Essential Coverage of Essential Places






